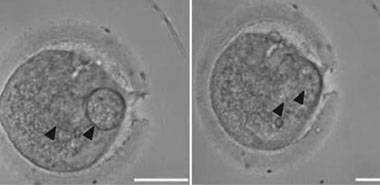
英国研究人员14日说,他们在世界上首次培育出拥有一个男性和两个女性遗传物质的受精卵,这一研究将来或许可以使母亲避免把有缺陷的线粒体遗传物质遗传给下一代。但也有人担心,这一研究存在伦理问题。
英国纽卡斯尔大学研究人员在当天的Nature杂志网络版上报告说,他们首次实现人类受精卵之间的DNA(脱氧核糖核酸)移植,从而获得一个拥有3个人遗传物质的受精卵,其中包括一名男性和一名女性的细胞核DNA,以及另一名女性的线粒体DNA。
负责这项研究的道格拉斯特恩布尔教授说:“我们在原则上证明:可以用相关技术来防止由线粒体引起的人类遗传疾病。”
研究人员强调指出,他们持有英国人工授精与胚胎学管理局颁发的执照并遵守了相关规定,没有违反人类胚胎研究的道德规范。
线粒体在人类细胞中的作用是提供能量,它所含有的遗传物质线粒体DNA只通过母系遗传,即只来源于卵细胞,与精子无关。由于未发生遗传交换,母亲的线粒体异常会导致许多遗传疾病,如Ⅱ型糖尿病等。据统计,大约每200个新生儿中就有1个带有可能致病的线粒体DNA变异。
在本次研究中,研究人员先从一对夫妇捐赠的受精卵中取出细胞核,然后将其植入另一个去除了细胞核的受精卵中。这个过程中几乎没有带入线粒体DNA,所获得的受精卵因此含有原父母的细胞核DNA和另一名女性的线粒体DNA。这个新的受精卵被培育了6到8天至胚泡阶段,这证实它可以正常发育。6到8天也是人工授精与胚胎学管理局规定的研究期限。
特恩布尔说,这种技术可以避免有缺陷的线粒体DNA遗传给下一代。他说:“这就像为笔记本电脑更换电池,硬盘上储存的信息不会发生改变。”
不过也有专家认为,不同来源的细胞核DNA和线粒体DNA不一定总是相容,存在产生冲突的风险。同时,由于存在伦理方面的争议,各国在法律上对类似研究都有一定限制,如英国目前不允许在人类子宫中培育使用上述方式产生的胚胎。
生物谷推荐原文出处:
Nature 14 April 2010 doi:10.1038
Therapeutic antibody targeting of individual Notch receptors
Yan Wu1,9, Carol Cain-Hom2,9, Lisa Choy2, Thijs J. Hagenbeek2, Gladys P. de Leon7, Yongmei Chen1, David Finkle4, Rayna Venook4, Xiumin Wu5, John Ridgway5, Dorreyah Schahin-Reed6, Graham J. Dow2,10, Amy Shelton2, Scott Stawicki1, Ryan J. Watts6, Jeff Zhang8, Robert Choy8, Peter Howard8, Lisa Kadyk8, Minhong Yan5, Jiping Zha3, Christopher A. Callahan3, Sarah G. Hymowitz7 " Christian W. Siebel2
Top of pageAbstractThe four receptors of the Notch family are widely expressed transmembrane proteins that function as key conduits through which mammalian cells communicate to regulate cell fate and growth1, 2. Ligand binding triggers a conformational change in the receptor negative regulatory region (NRR) that enables ADAM protease cleavage3, 4 at a juxtamembrane site that otherwise lies buried within the quiescent NRR5, 6. Subsequent intramembrane proteolysis catalysed by the γ-secretase complex liberates the intracellular domain (ICD) to initiate the downstream Notch transcriptional program. Aberrant signalling through each receptor has been linked to numerous diseases, particularly cancer7, making the Notch pathway a compelling target for new drugs. Although γ-secretase inhibitors (GSIs) have progressed into the clinic8, GSIs fail to distinguish individual Notch receptors, inhibit other signalling pathways9 and cause intestinal toxicity10, attributed to dual inhibition of Notch1 and 2 (ref. 11). To elucidate the discrete functions of Notch1 and Notch2 and develop clinically relevant inhibitors that reduce intestinal toxicity, we used phage display technology to generate highly specialized antibodies that specifically antagonize each receptor paralogue and yet cross-react with the human and mouse sequences, enabling the discrimination of Notch1 versus Notch2 function in human patients and rodent models. Our co-crystal structure shows that the inhibitory mechanism relies on stabilizing NRR quiescence. Selective blocking of Notch1 inhibits tumour growth in pre-clinical models through two mechanisms: inhibition of cancer cell growth and deregulation of angiogenesis. Whereas inhibition of Notch1 plus Notch2 causes severe intestinal toxicity, inhibition of either receptor alone reduces or avoids this effect, demonstrating a clear advantage over pan-Notch inhibitors. Our studies emphasize the value of paralogue-specific antagonists in dissecting the contributions of distinct Notch receptors to differentiation and disease and reveal the therapeutic promise in targeting Notch1 and Notch2 independently.